Abstract
Background and Methods: FMS-like tyrosine kinase 3 (FLT3) internal tandem duplication (FLT3-ITD) and tyrosine kinase domain mutation (FLT3-TKD) are types of mutations present in approximately 30% of patients with acute myeloid leukemia (AML). Currently, FLT3 inhibitors (FLT3i) are available in clinical practice, and the second-generation FLT3i, gilteritinib and quizartinib, are being used in Japan. However, the actual epidemiology of FLT3 mutations and co-existing gene alterations, particularly resistance mechanisms after FLT3i treatment, have not been thoroughly investigated in a Japanese population. Therefore, we conducted an actionable mutation profiling multicenter study, Hematologic Malignancies (HM)-SCREEN-Japan 01 (UMIN000035233), in which a comprehensive genomic assay was performed using the FoundationOne Heme (F1H) panel for patients with relapsed/refractory (R/R) AML and patients with newly diagnosed AML who were ineligible for standard chemotherapy (ND unfit). Paraffin-embedded bone marrow samples were used for next-generation sequencing (NGS) examination using the F1H panel. We analyzed the relationships between FLT3 gene mutations and other mutations and then chronologically evaluated the variant allele frequency (VAF) of gene mutations in the genomic profiles of patients with AML receiving FLT3i.
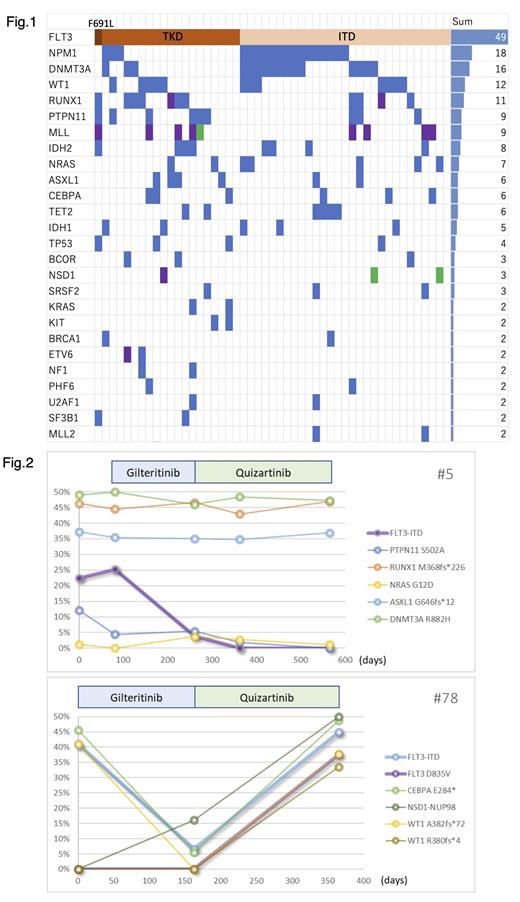
Results: Of the 171 patients who participated in this study, 49 (28.7%) had FLT3 mutations. FLT3-ITD and FLT3-TKD accounted for 59% and 43% of all cases of FLT3 mutations, respectively. Two patients (4%) were found to have dual mutations: one with FLT3-ITD plus FLT3-TKD and another with FLT3-ITD plus FLT3-F691L. Eight patients (4.5%) were found to have the FLT3-N676K mutation, which is sensitive to gilteritinib but undetectable by currently available PCR-based companion diagnostic tools in Japan. Frequently co-occurring mutations included those of NPM1 (37%), DNMT3A (33%), IDH1/IDH2 (27%), WT1 (24%), and RUNX1 (22%). Mutations in RAS pathway-related genes (e.g., KRAS, NRAS, and PTPN11) were observed in 15 patients (31%). No gene alteration showed statistically significant co-occurrence with the FLT3mutation. However, the median number of mutations that co-exist with FLT3-TKD was slightly higher than that of FLT3-ITD (four genes [3-5] vs. three genes [2-5]). Sequential changes in the VAF of each gene alteration were investigated in nine patients with FLT3 mutations who eventually gained resistance to FLT3i. It was suggested that there were various patterns in clone evolution. Some showed the acquisition of not only CBL or NRAS as RAS pathways, but also other driver mutations: one showed a persistent FLT3mutation, one showed FLT3-ITD plus FLT3-TKD, and one showed a newly acquired FLT3 mutation substituting an existing FLT3 mutation. We also found that founder mutations, such as the DNMT3Amutation, remain even after eradication of FLT3 mutation during treatment with FLT3i, which could be the cause of the outcome of complete remission with incomplete hematologic recovery.
Conclusions: This is the first report to analyze R/R and ND unfit AML cases in a Japanese cohort using F1H NGS, revealing a higher incidence of FLT3-ITD/TKD mutations than previously reported. Therefore, F1H mutational analyses for R/R and ND unfit AML patients harboring FLT3-ITD/TKD mutations may reveal novel therapeutic targets that are sensitive to FLT3i. Samples from these patients showed non-canonical gain-of-function mutations, such as N676K, S451F, V592D, and F691L, which could guide the selection of optimal anti-FLT3 therapies. In addition, longitudinal NGS analysis revealed clonal evolution in cases in which resistance to the FLT3i, gilteritinib and quizartinib were observed. Time-dependent analysis of allele frequencies can help evaluate the details of leukemia clonal evolution and provide optimal treatment options.
Figure Legends
Fig.1 Overview of gene mutations using F1H NGS analyses. The color of each column indicates the type of genetic mutation. Blue column; point mutation/insertion/deletion, green column; fusion gene, purple column; dual mutations.
Fig.2 The chronological changes of leukemic cells fractions bearing each gene mutations during treatment with FLT3 inhibitors, gilteritinib and quizartinib.
Shibayama: Otsuka: Honoraria; Pfizer: Honoraria; Bristol-Myers Squibb: Honoraria; Sanofi: Honoraria; Nippon Shinyaku: Honoraria; Fujimoto: Honoraria; Daiichi Sankyo: Speakers Bureau; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Chugai: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Research Funding; Novartis: Research Funding, Speakers Bureau; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AbbVie: Research Funding, Speakers Bureau; Takeda: Research Funding, Speakers Bureau; Ono: Research Funding, Speakers Bureau; Celgene: Research Funding; Mundi Pharma: Honoraria; Essentia Pharma Japan: Research Funding. Yamauchi: Otsuka: Research Funding; Ono Pharmaceutical: Honoraria; Pfizer: Honoraria, Research Funding; Chugai: Honoraria; Abbie: Research Funding; Astellas: Research Funding; Daiichi Sankyo: Research Funding; Solasia Pharma: Research Funding. Kondo: Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Bristol-Myers Squibb Company: Honoraria; Novartis Pharma KK: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Astellas Pharma Inc.: Consultancy, Honoraria; SANWA KAGAKU KENKYUSHO CO.,LTD.: Consultancy. Yamamoto: IQIVA/Genmab: Research Funding; Micron: Honoraria; Zenyaku: Honoraria, Research Funding; Yakult: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; SymBio: Honoraria, Research Funding; Solasia Pharma: Research Funding; Sanofi: Honoraria; Otsuka: Honoraria, Research Funding; Ono: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Nippon Shinyaku: Honoraria, Research Funding; Mundipharma: Research Funding; MSD: Honoraria; Meiji Seika Pharma: Consultancy, Honoraria, Research Funding; Kyowa Kirin: Honoraria; Janssen: Honoraria; HUYA: Consultancy; IQIVA/HUYA: Honoraria; IQIVA/Incyte: Research Funding; Eisai: Honoraria, Research Funding; Daiichi Sankyo: Honoraria; Chugai: Honoraria, Research Funding; Bristol-Myers Squibb/Celgene: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; ADC Therapeutics: Honoraria. Kuroda: Kyowa Kirin: Honoraria, Research Funding; Otsuka Pharmaceutical: Honoraria, Research Funding; Astellas Pharma: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; MSD: Research Funding; Abbvie: Consultancy, Honoraria; Ono Pharmaceutical: Honoraria, Research Funding; Eisai: Honoraria, Research Funding; Sysmex: Research Funding; Pfizer: Honoraria, Research Funding; Nippon Shinyaku: Honoraria, Research Funding; Shionogi: Research Funding; Asahi Kasei: Research Funding; Taiho Pharmaceutical: Research Funding; Fujimoto Pharmaceutical: Current Employment, Honoraria, Research Funding; Dainippon Sumitomo Pharma: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Chugai Pharmaceutical: Honoraria, Research Funding; Bristol-MyersSquibb: Consultancy, Honoraria, Research Funding; Janssen Pharmaceutical K.K: Consultancy. Usuki: Otsuka Pharmaceutical Co., Ltd.: Research Funding, Speakers Bureau; Novartis Pharma K.K.: Research Funding, Speakers Bureau; Ono Pharmaceutical Co., Ltd.: Research Funding, Speakers Bureau; Janssen Pharmaceutical K.K.: Research Funding; Celgene K.K.: Research Funding, Speakers Bureau; Takeda Pharmaceutical Co., Ltd.: Research Funding, Speakers Bureau; Nippon-Boehringer-Ingelheim Co., Ltd.: Research Funding; Mundipharma K.K.: Research Funding; Amgen-Astellas Biopharma K.K.: Research Funding; Nippon-Shinyaku Co., Ltd.: Research Funding, Speakers Bureau; Kyowa-Kirin Co., Ltd.: Research Funding, Speakers Bureau; Pfizer Japan Inc.: Research Funding, Speakers Bureau; Alexion Pharmaceuticals, Inc.: Research Funding, Speakers Bureau; Eisai Co., Ltd.: Speakers Bureau; MSD K.K.: Research Funding, Speakers Bureau; PharmaEssentia Japan KK: Research Funding, Speakers Bureau; Yakult Honsha Co., Ltd.: Research Funding, Speakers Bureau; Daiichi Sankyo Co., Ltd.: Research Funding, Speakers Bureau; Sumitomo-Dainippon Pharma Co., Ltd.: Research Funding; SymBio Pharmaceuticals Ltd.: Research Funding, Speakers Bureau; Gilead Sciences, Inc.: Research Funding; Bristol-Myers-Squibb K.K.: Research Funding, Speakers Bureau; Apellis Pharmaceuticals, Inc.: Research Funding; AbbVie GK: Research Funding, Speakers Bureau; Astellas Pharma Inc.: Research Funding, Speakers Bureau; Incyte Biosciences Japan G.K.: Research Funding; Chugai Pharmaceutical Co., Ltd.: Research Funding, Speakers Bureau; Sanofi K.K.: Speakers Bureau; Amgen K.K.: Research Funding. Yoshimitsu: Novartis: Honoraria; Takeda: Honoraria; Sanofi: Honoraria. Ishitsuka: Eisai: Other: Personal fees, Research Funding; Sumitomo Dainippon Pharma: Other: Personal fees, Research Funding; Genzyme: Other: Personal fees; Astellas Pharma: Other: Personal fees, Research Funding; Pfizer: Other: Personal fees; Novartis: Other: Personal fees; Janssen Pharmaceuticals: Other: Personal fees; Taiho Pharmaceuticals: Other: Personal fees, Research Funding; Mundipharma: Other: Personal fees; Takeda: Other: Personal fees, Research Funding; BMS: Other; Chugai Pharmaceutical: Honoraria, Other: Personal fees, Research Funding; Celgene: Honoraria, Other: Personal fees; Ono Pharmaceutical: Other: Personal fees, Research Funding; Kyowa Kirin: Other: Personal fees, Research Funding; Daiichi Sankyo: Consultancy, Other: Personal fees; MSD: Research Funding; Teijin Pharma: Research Funding; Otsuka Pharmaceutical: Other: Personal fees; Shire: Other; Mochida: Other: Personal fees, Research Funding; Asahi kasei: Research Funding; Eli Lilly: Research Funding; Huya Japan: Other: Personal fees. Ono: Kyowa Kirin Co., Ltd.: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Janssen Pharmaceutical K.K: Honoraria; Eisai Co., Ltd.: Honoraria; Astellas Pharma Inc.: Honoraria; Takeda Pharmaceutical Company Limited.: Honoraria; ONO PHARMACEUTICAL CO., LTD.: Honoraria, Research Funding; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Pfizer Japan Inc.: Honoraria; Bristol-Myers Squibb Company: Honoraria; Novartis Pharma KK: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding; DAIICHI SANKYO COMPANY, LIMITED.: Honoraria; Mundipharma K.K.: Honoraria; TAIHO PHARMACEUTICAL CO., LTD.: Research Funding; Merck Sharp & Dohme: Honoraria, Research Funding. Takahashi: Kyowahakko-Kirin: Research Funding; Toyamakagaku: Research Funding; Otsuka Pharmaceutical: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Chugai: Research Funding; Eizai: Research Funding; Asahikasei: Research Funding; Ono: Research Funding. Iyama: SymBio Pharmaceuticals: Research Funding; Astellas: Honoraria; CSL Behring: Honoraria; Daiichi Sankyo: Honoraria; Otsuka Pharmaceuticals Factory: Honoraria; Otsuka Pharmaceuticals Factory: Honoraria; MSD: Research Funding; Nippon Shinyaku: Honoraria; Novartis: Honoraria; Otsuka: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Alexion Pharmaceuticals: Honoraria, Research Funding. Izutsu: Allergan Japan: Honoraria; Symbio: Honoraria, Research Funding; Pfizer: Research Funding; Ono: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; MSD: Research Funding; Janssen: Honoraria, Research Funding; Incyte: Research Funding; Genmab: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; Beigene: Research Funding; Bayer: Research Funding; AstraZeneca: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Yakult: Research Funding; Takeda Pharmaceutical: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; HUYA Bioscience International: Research Funding; Eisai: Honoraria, Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; FUJI FILM Toyama Chemical: Honoraria. Minami: Bristol-Myers Squibb Company: Honoraria; Pfizer Japan Inc.: Honoraria; Takeda: Honoraria; Novartis Pharma KK: Honoraria; Astellas: Honoraria; Ono: Research Funding; CMIC: Research Funding.